This page is under construction, though readable.
The wonder material graphene was postulated in 1947, announced in 1962 and we had the term in 1986. It was 2003/4 before its production was demonstrated. [1] Graphene is one of several special cases of the structure of carbon, in which we could include diamond, carbon nanotubes and fullerene / buckyballs. Between 2014 and 2017, commercial production began (in the UK).
Think of graphene as a thin film of graphite, quite possibly a single atom thick. There's a wonderful technique, the scotch tape technique which allows transfer of the thin layer to some other surface such as silicon. In essence, 3D-graphite has thin layers pulled off it. As a layer a single atom thick it is a crystal lattice of high-quality, meaning it is uniform in thickness and continuity. Among the properties of this surprising material is that it conducts electricity well, in ways that are themselves a surprise and that suggest this would make a substitute for silicon chips, especially if a graphene coating could be formed on a suitable substrate so that it behaves as a semiconductor. This suggests we could build circuit boards at the nanoscale, which would be very, very fast, clock speeds of terahertz (where 2020 fast chips are around 5 GHz). [3] and see comment at [7].
This would herald a new age in computing, already coined as the post-silicon age [5]. That will keep us up with Moore's Law, that says transistors per unit area will double every two years, though I don't see that this will go much beyond 2025 (at the moment).
The properties of graphene are not merely electric; it is also extremely strong, very flexible and elastic; it conducts heat very well. Detail at [7]. Forming nanotubes for graphene produced a surge in possibilities, tempered somewhat by similarities to asbestos in its behaviour.
In graphene, carbon atoms link up in an orderly honeycomb pattern, each atom sharing electrons with three neighbouring carbon atoms. That structure allows any added electrons to move speedily across its surface. Ordinarily, a single electron might move through a conducting metal like copper at 1.2 inches per minute (given a 12-gauge wire with 10 amps of electricity). But in early experiments on graphene, electrons zipped along at 2.34 billion inches per minute — which could make for electronics that charge in just a few minutes and eventually in a matter of seconds. [6]
An investment opportunity, but one that perhaps should already have been taken.
Staring hard at a crystal ball, I can't help but wonder if graphene might allow a strong surface skin that somehow is also substantial load-bearing structure and sufficiently clever electronically that the surface could be screen, heading towards that point at which an object can show a distant observer what is behind the object, thus appearing optically invisible. Or a more general vessel, which we call a ship (water, air or space and their boundaries). If a very thin layer could be a chip, we're not so very far from the StarGate notion of the replicators. Very fast computing in a thin flexible shell is also suitable for enhancement to people (imagine a hat/helmet with serious processing capacity, doubling perhaps as looking like hair (yes please!!).
The non-carbon atomic skins (it's my term and I'm using it) all seem (now) to have chemical issues, though I noted that several are saved when sandwiched by skins of boron; I see this as worthy of exploration where the new science effects on offer are different from graphene and I wonder further at the possibility of multiple layered sandwiches (new word needed; Carbonwich? Ceewich?) having dramatic properties including significant computing power and structural strength. [8-11], read after writing this.
To-Do List
Rewrite last para.
Clear confusion in media between graphene (expensive) and graphene oxide (relatively cheap). [13] First we must establish what is graphene or graphite. It is generally accepted that 2D sheets that are less than 10 layers thick can be called graphene. There are 3 main types of graphene: Graphene films which are typically made by CVD, ALD, or Epitaxial growth, Graphene Oxide which is exfoliated by reacting graphite in sulphuric acid with potassium permanganate in a highly exothermic reaction, & Graphene Nanoplatelets which can be plasma exfoliated or chemically exfoliated, ball milled, or thermally shocked and sheared to product stacks of graphene nanoplatelets. How to chose between graphene and graphene oxide? It depends on your application. If you want electronic properties to make a sensor or device or improved mechanical properties, we recommend CVD graphene, or RGO. If you want good dispersion and solution processing, we recommend normal GO.
Not understood: the use of band and band-gap in chip design. This applies to semiconductors; a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in electron volts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electronbound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move in the solid; however, if some electrons transfer from the valence to the conduction band, then current can flow (see carrier generation and recombination). Therefore, the band gap is a major factor determining the electrical conductivity of a solid. Substances with large band gaps are generally insulators, those with smaller band gaps are semiconductors, while conductors either have very small band gaps or none, because the valence and conduction bands overlap. How chemistry (and solid-state physics) has moved since I was at school; this makes me feel utterly out of touch and I want a source that introduces these ideas in a sensible order. The diagram helped me, and led me to ask about interatomic distances in the different forms of carbon. The a shown is for diamond at about 0.357nm, while for graphite that figure is around 0.246nm. In graphene, 0,142nm and 0.335 between layers in the 3-d form. (researchgate and wikipedia).
Question: if conduction applies to electricity (and heat?), what does rising energy with smaller interatomic distance mean? Does it mean that there is increased electron mobility [in graphene (15,000, 0.246) over graphite (250,000 0.142) say]. Does that also mean everything is hotter? [No, not necessarily]
Conventional semiconductors like silicon have a bandgap in the range of 1 - 1.5 electronvolt (eV), whereas wide-bandgap materials have bandgaps in the range of 2 - 4 eV. Generally, wide-bandgap semiconductors have electronic properties which fall in between those of conventional semiconductors and insulators. Wikipedia
Look up 'fitness patch',
Graphene oxide [GO] is a single-atomic layered material, made by the powerful oxidation of graphite, which is cheap and abundant. Graphene oxide is an oxidized form of graphene, laced with oxygen-containing groups. It is considered easy to process since it is dispersible in water (and other solvents), and it can even be used to make graphene. Graphene oxide is not a good conductor, but processes exist to augment its properties. It is commonly sold in powder form, dispersed, or as a coating on substrates.
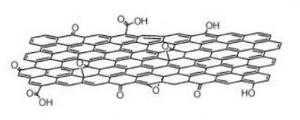
Graphene oxide is synthesized using four basic methods: Staudenmaier, Hofmann, Brodie and Hummers. Many variations of these methods exist, with improvements constantly being explored to achieve better results and cheaper processes. The effectiveness of an oxidation process is often evaluated by the carbon/oxygen ratios of the graphene oxide.
Graphene Oxide films can be deposited on essentially any substrate, and later converted into a conductor. This is why GO is especially fit for use in the production of transparent conductive films, like the ones used for flexible electronics, solar cells, chemical sensors and more. GO is even studied as a tin-oxide (ITO) replacement in batteries and touch screens.
Graphene Oxide has a high surface area, and so it can be fit for use as electrode material for batteries, capacitors and solar cells. Graphene Oxide is cheaper and easier to manufacture than graphene, and so may enter mass production and use sooner.
GO can easily be mixed with different polymers and other materials, and enhance properties of composite materials like tensile strength, elasticity, conductivity and more. In solid form, Graphene Oxide flakes attach one to another to form thin and stable flat structures that can be folded, wrinkled, and stretched. Such Graphene Oxide structures can be used for applications like hydrogen storage, ion conductors and nanofiltration membranes.
Then there is reduced graphene oxide, rGO [12]
Fast batteries - Huawei 2016, HowTo
Would bags of graphene decay? Does graphene decay at all? Could graphene displace plastic well or badly? What about embedding graphene in structure such as concrete, which could conduct heat away (to some useful place like a heat pump power generator), or provide heating and at the same time move electric power around.
How does price of graphene affect the possibilities? Since it is appearing in wearables, that implies its cost is already not preventatively expensive. Factors attaching to cost, at about $100 per gramme. Target has to be under $1/g. Graphene oxide is alrady less expensive, $75-225/g source
How are the medical applications working out? Even [11] is pretty quiet about this. Graphene contact lens?
Cars? Look at the Briggs (UK)
Wearable tickets - printed electronics - internet of things effect. Article. (diagram too) [15]
DJS 20210127
[1] https://en.wikipedia.org/wiki/History_of_graphene. I think we will be taught that this occurred at Manchester University in 2003, earning 2010 Nobel prizes for Andre Geim and Konstantin Novoselov, who were both knighted in 2012. Geim has also produced gecko tape, which looks to suggest that Spiderman may become a reality.
[2] https://www.aps.org/publications/apsnews/200910/physicshistory.cfm
[3] https://www.photonicsviews.com/a-new-ultra-high-speed-terahertz-chip/ the latest fast chip I found.
[4] IBM says they've built a graphene chip. https://www.dcncorp.com/news/ibm_graphene-chip-faster-using-standard-cmos-processes/ Also https://www.extremetech.com/extreme/175727-ibm-builds-graphene-chip-thats-10000-times-faster-using-standard-cmos-processes?goback=%2Egde_2245236_member_5837731363922411524 A short, easy read. Original article: https://www.nature.com/articles/ncomms4086 One can envision that high-performance graphene RF circuits will be directly built on top of high-density Si CMOS logic circuits to form an extremely low-cost, ultra-compact communication system.
[5] https://www.chemistryworld.com/news/post-silicon-age-dawns-as-carbon-nanotube-transistors-made-in-chip-factories/4011927.article CNT transistors; roll graphene tightly and a band gap forms (?) so that it does behave like a semiconductor. This is often described as using nanotubes to make transistors.
[6] https://www.discovermagazine.com/the-sciences/move-over-graphene-next-gen-2d-materials-could-revolutionize-technology discusses other single layer atomic skins (my phrase). Hence: phosphorene, which has many qualities in common with graphene though it behaves more easily as a semiconductor and does odd things with light at infra-red wavelengths suggesting a future in fibre comms and solar cells; Borophene is very flexible, transparent, electrically conductive and a similar layer of boron nitride is a good insulator, so sandwiches of BN-B-BN have possibilities; chromium triiodide, CrI₃, generates a magnetic field and again needs to be sandwiched between layers of BN to be usable – the magnetic effect suggests possible uses in computing memory and storage. There's a future for physicists in atomically thin sheets, atomic skins. Triiodide, a rare occurrence of a doubled letter-i. Though at the end, as in leylandii, is not so unusual. Skiing, taxiing, grafitiing, shanghaiing, foliicolous, zombiisms.
[7] https://iopscience.iop.org/article/10.1149/2162-8777/abbb6f A good source, achieving what I wanted to know.
Graphene [has] better physical properties [when] compared to other materials, such as high thermal conductivity (5000 Wm−1 K−1),7 high electron mobility (250,000 cm2V−1 s−1),8 high Young modulus values (1.0 TPa),9large surface area (2630 m2 g−1),10 and better electrical conductivity and optical transmittance.11 I found this meaningless until I looked up the physical constants (how do you do that these days, I used to have a wonderful book of Father's that did this);
Thermal conductivity: aluminium 237 copper 401, diamond 1000 graphene 5000Wm⁻¹K⁻¹ wikipedia.
Electron mobility: GaN 800, crystalline silicon 1400, graphite 15000 (source), carbon nanotubes, 79000, graphene 250,000 cm²V⁻¹s⁻¹ wikipedia When I hunted further on that 250000 figure I found that is theoretical (Chen et al, 2008). See here, article by BM Nichols, 2D Materials, 2016, but that electronmobility of 70,000 cm²V⁻¹s⁻¹ was achieved at room temperatures. Almost readable.
Young's modulus (approx): brass 100, wrought iron and steel 200, tungsten carbide 550, graphene 1000, diamond 1100 GPa. wikipedia
Large surface area (specific surface area): alumina as catalytic support 200, fine clay montmorillonite 800, Faujesite catalyst 900, activated carbon 500-3000, graphene 2630, metal organic framework 7140 m²g⁻¹ wikipedia
Electrical conductivity (Ohm-metres x 10⁻⁹) graphene (by volume) 12, silver 16, copper 17, Gold 24 Look. Confused.
Optical transmittance: this depends on the wavelength of the light under consideration. graphene (presumably looking to see if it will replace phone screens and like uses). single layer graphene showed a transmittance of 97.49% at 550 nm wavelength, Optical glass transmits light well (over 99%) from about 375-1275 nm (visible light is 380-700nm; Infra-red is 780-1000 The infrared spectrum can itself be divided into three distinct categories: near-infrared or NIR (wavelength: 0.75 – 1.4 micrometers), short wavelength infrared or SWIR (wavelength: 1.4 – 3 micrometers), mid wavelength infrared or MWIR (wavelength: 3 – 8 micrometers), long wavelength infrared or LWIR (8 – 15 micrometers), and far-infrared or FIR (wavelength: 15 – 1000 micrometers). I'm sorry, that is not three but five bands. Messy topic, but one might ask what range certain sorts of sensor work in. Source... PIR detectors...
[8] https://en.wikipedia.org/wiki/Potential_applications_of_graphene Read after writing. I omitted the solar cells; I assumed that price would fall to competitive levels with other materials. Article gives good coverage (but what would I know; I call it good because it looked further than I did). How permeable to water is graphene? Could it make a light bulb?
[9] https://web.archive.org/web/20130320001240/http://bucky-central.me.utexas.edu/RuoffsPDFs/179.pdf on graphene-based ultra capacitors. The surface area of a single graphene sheet is 2630 m2/g, substantially higher than values derived from BET surface area measurements of activated carbons used in current electrochemical double layer capacitors. Our group has pioneered a new carbon material that we call chemically modified graphene (CMG). CMG materials are made from 1-atom thick sheets of carbon, functionalized as needed, and here we demonstrate in an ultracapacitor cell their performance. Specific capacitances of 135 and 99 F/g in aqueous and organic electrolytes, respectively, have been measured. In addition, high electrical conductivity gives these materials consistently good performance over a wide range of voltage scan rates. These encouraging results illustrate the exciting potential for high performance, electrical energy storage devices based on this new class of carbon material.
[10] https://www.graphene.manchester.ac.uk/learn/applications/ links to several other pages. Among these, that graphene oxide—a different material from graphene—can be used as membranes (between fluids). How about graphene packaging? Graphene water filters? Gas separators? desalination? If all that, how about inside batteries, where a membrane is often wanted between the electrodes?
[11] https://www.techradar.com/uk/news/dummy-40-ways-graphene-is-about-to-change-your-life
[12] Graphene-info https://www.graphene-info.com/ All you wanted to know (they think). Example, air purifiers (refers to germs, not viruses) https://youtu.be/p9IMn_uTv2Q
[13] https://www.cheaptubes.com/product-category/graphene-oxide/
[14] https://www.sciencedirect.com/topics/engineering/graphene Science gobbledegook mostly. The test is that if you can read material like this, it is okay for you. If you can't, the implication is that you shouldn't even be trying. I resent that. I tried reading the whole, some of which was actually interesting: Thin flakes of graphite were repeatedly peeled off the mesas using scotch tape. Then, we selected those flakes that were so thin as to remain practically invisible under an optical microscope. They were released by dissolving scotch’s visco-elastic layer in acetone and captured on a Si wafer. Large flakes often appeared crumpled. [....] We emphasize that despite many steps involved in the film and device microfabrication, the described procedures were found to be highly reproducible, except for the peeling-off process that in the end relies on manual selection of a most suitable thin flake.
[15] https://iopscience.iop.org/article/10.1149/1945-7111/ab67a4 Review—Wearable Graphene Devices for Sensing. Variably readable; images interesting.