At this point I'm going to look at concrete as a specific industrial process, concrete production being such a small part of 'the anthropocentric generation of greenhouse gases'. Now there's a phrase; "Look what mess I done, Ma!" might just as well be used.
When we make cement (and I mean cement, not concrete) we basically take a carbonate and turn it back to an oxide. Typically we begin with a rock that is mostly limestone, crush it to a powder, heat it extremely (1500ºC) to drive off the CO₂ and keep it dry. [6] and [7] explain this. When making concrete, using the cement mixed with granulated rock such as sand, CO₂ is removed from the atmosphere as the mix turns back into calcium carbonate (think 'rock', or a form of it).
There is a second form of cement, hydraulic cement (Portland cement is one such) that are mixtures of silicates and oxides. Hardening of hydraulic cements occurs by hydration; the chemistry is sufficiently complicated to represent a research topic and is one with a future, even as concrete becomes phased out due to what we might call green pressures. 6 Source [8] is a long read. The ecological concern is that, while concrete is very useful and surprisingly cheap, the environmental 'costs' are significant. I assume that the net effect is to generate more CO₂ from the chemical conversion of rock back to rock ([8] says 60% of that CO₂ attributed to cement / concrete) and from the consumption of power to make this occur (source [8] says 40%, of course) — the crushing, grinding, heating, mixing and delivery, most of which is explained as the burning of fuel. The percentage of CO₂ that this is said to represent varies from 4% to 10% and I failed to distinguish how these figures were different. 4.
Quoting [8] Cement manufacture causes environmental impacts at all stages of the process. These include emissions of airborne pollution in the form of dust, gases, noise and vibration when operating machinery and during blasting in quarries, and damage to countryside from quarrying. Equipment to reduce dust emissions during quarrying and manufacture of cement is widely used, and equipment to trap and separate exhaust gases are coming into increased use. Environmental protection also includes the re-integration of quarries into the countryside after they have been closed down by returning them to nature or re-cultivating them. Source [9], p31 et seq., explains that this means that cement clinker production is the largest of non-combustion sources of CO₂ from industrial manufacturing, contributing to about 4.8% of the total global emissions in 2013. Fuel combustion emissions of CO₂ related to cement production are of approximately the same level, so, in total, cement production accounts for roughly 9.5% of global CO₂ emissions. 5
This source explains that emissions are not directly proportional to cement production level, since the fraction of clinker — in this industry the main source of CO2 emissions — in cement tends to decrease over time. (....) Average clinker fractions in global cement production have decreased to between 70% and 80%, compared to nearly 95% for Portland cement with proportional decrease in CO₂ emissions per tonne of cement produced. Both non-combustion and combustion emissions from cement production occur during the clinker production process, not during the mixing of the cement clinker. This has resulted in about 20% decrease in CO₂ emissions per tonne of cement produced, compared to the 1980s. At that time, it was not common practice to blend cement clinker with much other mixing material, such as fly ash from coal-fired power plants or blast furnace slag. According to EDGAR 4.2 data, this yielded an annual decrease of 250 million tonnes in CO₂ emissions, compared to the reference case of Portland cement production. Moreover, a similar amount has been reduced in fuel combustion for cement production and related CO₂ emissions.
So we might conclude that the industry has been working to reduce its emissions, just as we might as easily say this is entirely from self-interest in reducing costs. We might even say that the environmental sensitivity is being recognised. Quite how decisions are reached is as much political as economic. In the end, we have a situation where even though total cement production has continued to rise, it looks as though the effect of this has not increased. That must be a good thing.
Paraphrasing wikipedia [6]; cement manufacture generates CO₂ from
(i) reducing limestone to the oxide (lime, quicklime, CaO). This is around 0.5kg (0.47 to 0.54) of CO₂ per kg of cement, worldwide.
(ii) the combustion in the kiln, which obviously will vary with the plant, from 0.24 to 0.65 (kg CO₂ per kg cement). In the UK, 0.3.
(ii) from the transport of cement, including distribution. By comparison, trivial sums, 0.002-0.005 kg CO₂ per kg cement.
This ignores or omits the CO₂ from associated electricity generation/consumption, around 90-150kWh per tonne cement, where 1 kWh per tonne equates to 0.01 kg CO₂ per kg of finished cement if the electricity is coal-generated. 7 I think that amounts to an addition tot the 'kiln' figure at (ii) above so 0.24-0.65 might move to 0.3-0.8. The thrust of innovation for the future is to reduce sources 1 and 2 by modification of the chemistry of cement, by the use of wastes, and by adopting more efficient processes. Although cement manufacturing is clearly a very large CO2 emitter, concrete (of which cement makes up about 15%) compares quite favourably with other building systems in this regard.Yes, I'd like to see a citation and I'd follow up on that.
Alternative concretes
alkali-activated cements including geopolymeric cements (e.g. Zeobond/e-crete, Blue World Crete/Geo-Blue Crete, banah/banahCEM)
low energy CSA-belite cements (e.g. Aether);
cements based on magnesium oxide derived from carbonates or from silicates (e.g. Eco-cement, Calix/Novacem);
'ecocement' based on municipal solid waste incinerator ash (MSWIA);
thermoplastic carbon-based cements (e.g. C-Fix cement).
Concrete is a surprisingly versatile material and we use it an awful lot, so let's look first at what we might do to further reduce the energy consumption and the CO₂ generation attached to its manufacture. [11] applies. As yet, none of the alternatives yet proffered are realistic alternatives.
• We can attempt to use less concrete, but we do have a need for a flexible equivalent to stone, so even if we design anew to use less concrete, we still need a substitute.
• We can take clinker from other processes (fly ash is one such), basically substituting for the clinker element. [13] classifies these. One issue is that limestone is plentiful, whereas alternatives are not. Look at [17] for cement part-substitutes. Might 'cement supplements' be a better term ? Ways of stretching Portland cement, anyway.
• We can substitute for the calcium carbonate with silicates or phosphates. One plentiful alternative is magnesium silicates, which contain essentially no chemically combined (“fossil”) CO2 and thus have the potential to make very carbon-efficient MgO-based products if suitably energy-efficient extraction technologies can be invented to separate the MgO in a reasonably pure and reactive form. 8. The attraction is that if reactive anhydrous calcium silicate and aluminate compounds were abundantly available in nature, instead of calcium carbonates, it would be possible to make binders that could absorb more CO2 by carbonation during use than emitted by the fuel required for the manufacturing process. One would thus be able to make “carbon-negative” PCC-based binders which could be used in construction while serving to reduce net global CO2 emissions. The sources we would wish to use are calcium silicate wastes from other processes; in general these create local solutions, not global ones. The same general complaint—global scarcity—applies to magnesium and calcium phosphates.
• We can take old concrete and return it to use. It is the cost of transport that tends to remove this from consideration, though another factor is contamination.
• We can shift to alkali-activated binders. 9 [14] is essentially a material that gains strength by means of a chemical reaction between a source of alkali, commonly an alkali silicate solution, and an aluminate-rich material. As well as being highly sustainable, largely composed of industrial by-products already familiar to the concrete producer, such as fly ash and blast furnace slag, they can exhibit performance characteristics beyond those of conventional Portland cement-based materials. Enhanced chemical, wear, and temperature resistance have all been shown to be achievable. Bearing more resemblance to a natural stone than a cement, these materials promise low-carbon, high-performance alternatives to current binder technology. See
• We can substitute plant material for the clinker — typically fibres from non-wood plants such as sisal, green coconut, bamboo, sugarcane bagasse, curauá, and jute. [15] Hempcrete is an example, seen at [16, § 4]. We can also add, besides hempcrete, ashcrete (fly ash from power stations, see cenocell), timbercrete (sawdust), ferrock (steel dust), plascrete (recycled plastic). Grasscrete is a false flag in this group, since it applies to open weave brickwork in which grass is encouraged to grow in the gaps — for driveways and similar. Of course, if one ash can be used, so can others, but whether this is supplementing or replacing the cement depends on the chemistry. One example of this is rice-crete (my term), which is an example claiming to be carbon neutral — and that might well be true if the transport of the material is minimal.
• We can use different materials entirely for some uses of concrete. See [16] for a brief overview. Straw bales, rammed earth, timber (including bamboo). Mycelium -based material is promising but it is not sufficiently tested as yet for long-term use.
I note that the many available articles illustrate a confusion about what the constituents of concrete are and of the chemistry of concretes. The terminology often aids the confusion. Does clinker form the cement, the binder for whatever aggregate is used? Is the binder confused with the reinforcement? Do even some of the writers see the last six letters of reinforcement and add to their own confusion? Clarification: the biggest component by volume is aggregate, where the issue is particle size (coarse or fine, would you believe). This is the component most easily substituted, so the issues are whether the binder can in some sense 'grip' the aggregate, whose other physical properties will determine whether the result will have adequate tensile strength, compressive strength. Then there is the cement, which is the binder of the aggregate and the necessary piece of chemistry. The hydraulic cements described at the top take in water (they hydrate) and solidify. 10 Obviously, water is added and this has two purposes; the hydration of the cement and the workability of the material while reasonably fluid. We can choose to add fibres, which will affect both the plastic and hard states–we might include steel reinforcement here, since it serves much the same purpose. Typical fibres beyond steel are glass and polypropylene. Last we might choose to add admixtures; like the fibres, these affect the plastic and hardened states, but are far smaller in quantity and more varied in effect. See [18] for a list.
DJS 20190705
Figure 3, from [5] with a centred x-axis, for which you should use the link to read the detail. https://www.earth-syst-sci-data.net/10/2141/2018/essd-10-2141-2018-f03.png
Footnote numbering continues from essay 282.
4 This is yet another case of numbers failing to be properly communicated. We hear the number but fail to explain the context sufficiently well to be able to say what the figure is. 4% of what, exactly?
5 Cement clinker is the result of the extreme heating of the rock mix. Clinker is a term for rock that has been heated so much it sinters; very dry, very hard, no liquefaction. [10] may help. Does the clinker participate in the chemistry? [6] says it does, but fails to explain the chemistry that does occur. [19] indicates that 75% of Portland cement is silicate (powdered clinker, calcium trisilicate and disilicate). Chemistry follows:
Tricalcium silicate + Water--->Calcium silicate hydrate + Calcium hydroxide + heat 2 Ca3SiO5 + 7 H2O ---> 3 CaO.2SiO2.4H2O + 3 Ca(OH)2 + 173.6kJ
Dicalcium silicate + Water--->Calcium silicate hydrate + Calcium hydroxide + heat 2 Ca2SiO4 + 5 H2O---> 3 CaO.2SiO2.4H2O + Ca(OH)2 + 58.6 kJ
One assumes that the non-hydraulic reaction also occurs, Ca(OH)₂(s) + CO2(g) → CaCO₃(s) + H₂O(l) - 113kJ
6 Can it really still be true that the most important property of one of the central and widespread materials of 20th and 21st century civilisation, tightly and widely formulated, and composed only of inorganic molecules, is still, more than 150 years after its formulation, only partly understood? From the talk section on the wiki site [6]. I agree, it does seem so.
7 Source statement 90-150 kWh per tonne cement, equivalent to 0.09-0.15 kg CO₂ per kg finished cement if the electricity is coal-generated." I question the accuracy of this. I found the source figure 150kWh/t cement here. That led to far too many places where screaming and shouting of the political greening displaces the raw information. In the UK we have 0.527 kg of CO₂/kWh (google CO2 per kwh electricity) and that compares well with (same source) 1kg per unit for coal worldwide. That last figures confirms the statement at the beginning of this paragraph: coal generation makes a kg of CO₂ per kWh and 150kWh/t cement represents 150kg of CO₂/t, which fits exactly with 0.15 kg CO₂ per kg finished cement if the electricity is coal-generated.
Just the conversion of ore to clinker is around 5 GJ/t =>1400kWh/t, which conflicts. See Talk at [6]
8 from [13] Well-known technologies already exist for using MgO to make binders suitable for construction applications. “Sorel” cements, based on mixtures of powdered MgO with concentrated solutions of magnesium chloride or sulfate, have been known for well over a century and have some applications in construction, but are only suitable for use in dry environments. Magnesium phosphate cements, based on mixtures of powdered MgO with concentrated solutions of ammonium or potassium dihydrogen phosphate, have also been used in specialty construction applications for many decades. They have good water-resistance, rapid strength gains and high ultimate strengths. However, neither of these MgO-based binder technologies is currently suitable for general construction applications, mainly due to the scarcity of the raw materials compared to those required for PCC, but also because the manufacture of the main “clinker” component for both of these technologies, MgO, involves a very energy- and CO2-intensive production process. Currently, the main source of MgO is calcination of natural magnesite, (MgCO3, a very scarce mineral compared to limestone) which results in total CO2 emissions of the order of 1.55 tons of CO2 per tonne of MgO produced (see detailed calculation in the appendix). Some MgO is also produced from brines or seawater, but with an even higher carbon footprint. Because the carbon footprint of MgO produced in these ways is almost a factor of two higher than for PCC, we discount the use of conventionally-sourced MgO-based hydraulic binders in concrete as a route to improved carbon-efficiency in construction. In order to circumvent this problem, certain groups have proposed the use of carbonation hardening instead of hydration hardening as a way of reducing the carbon footprint of MgO based-binders [4], and this is indeed possible; but at the moment it seems to us that such binders offer no special advantages relative to the calcium-based carbonatable binders discussed in detail in section (C) of this paper, and still suffer from the relative scarcity of the main raw material [5].
9 In general the topic of concrete can be described as a geopolymer, a mobile, manipulable material that subsequently sets to a rock-like equivalent. Bitumen might be one such. Many industrial wastes produce a clinker equivalent for which the addition of a suitable binder will provide an attractive—from several aspects—alternative to mass concrete.
10 Originally, cement meant Portland cement and was a contraction of the longer term. Cement has come to mean whatever binder is being used. We've done much the same with 'plastic', which was originally a descriptor of behaviour and has become the noun for a whole class of materials, confounded with terms such as 'rigid plastic', surely an oxymoron.
[2] https://whatsyourimpact.org/greenhouse-gases/carbon-dioxide-emissions
[3] https://www.wri.org/blog/2018/12/new-global-co2-emissions-numbers-are-they-re-not-good
[4] https://www.globalcarbonproject.org/carbonbudget/
[5] https://www.earth-syst-sci-data.net/10/2141/2018/
[6] https://en.wikipedia.org/wiki/Cement
[7] https://www.cement.org/cement-concrete-applications/how-cement-is-made
[10] https://www.understanding-cement.com/clinker.html
[11] https://cement.mineralproducts.org/documents/FS_12_Novel_cements_low_energy_low_carbon_cements.pdf
[12] https://core.ac.uk/download/pdf/82057275.pdf paper explaining something of the energy required to calcinate limestone to quicklime. Might produce a thread of investigation towards more energy-efficient route to producing lime.
[13] https://www.sciencedirect.com/science/article/pii/S000888461630775X
[14] https://www.sciencedirect.com/topics/engineering/alternative-binder
[15] https://www.sciencedirect.com/science/article/pii/B9780081003701000196
[16] https://inhabitat.com/11-green-building-materials-that-are-way-better-than-concrete/
[17] http://www.greenspec.co.uk/building-design/concrete-cement-substitutes/
[18] https://www.hanson.co.uk/en/ready-mixed-concrete/technical-information/concrete-constituents
[19] http://matse1.matse.illinois.edu/concrete/prin.html
This from [20]
Enthalpy of formation for selected substances- CO2(g) ΔH f∘= −393 kJ/mol; Ca(OH)2(s) ΔH f∘= −987 kJ/mol; H2O(l)
ΔH f∘= −286 kJ/mol; CaCO3(s) ΔH f∘= −1207 kJ/mol
We’re being asked to determine the standard enthalpy change (ΔH˚rxn) for the balanced reaction:
Ca(OH)2(s) + CO2(g) → CaCO3(s) + H2O(l)
Recall that ΔH˚rxn can be calculated from the enthalpy of formation (ΔH˚f) of the reactants and products involved:
 /p>
/p>
We’re given the following ΔH˚f values:
ΔH˚f Ca(OH)2(g) = −987 kJ/mol ΔH˚f CaCO3(l) = −1207 kJ/mol
ΔH˚f CO2(g) = −393 kJ/mol ΔH˚f H2O(l) = −286 kJ/mol
Take note that we need to multiply each ΔH˚f by the stoichiometric coefficient. Solving for ΔH˚rxn:
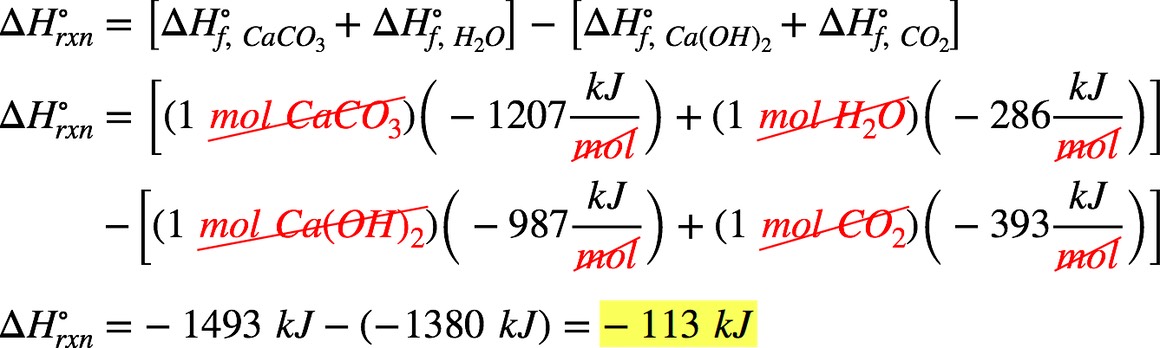 /p>
/p>
The ΔH˚rxn of the reaction is –113 kJ.